
| Catalog Number | Product | Size | Price | |
|---|---|---|---|---|
| C3039 | Human EGFR-CT26 Stable Cell Line | 2 vials | $6950 | Order |
| Catalog Number | C3039 |
|---|---|
| Cell Line Name | Human EGFR-CT26 Stable Cell Line |
| Accession Number | X00588.1 |
| Host Cell | Adherent CHO-K1 |
| Quantity | Two vials of frozen cells (2x106 per vial) |
| Culture Medium | RPMI with 10% FBS, 10 ug/ml puromycin |
| Freezing Medium | 90% FBS and 10% DMSO |
| Storage | Liquid nitrogen upon receipt |
| Product Datasheet: | Download PDF |
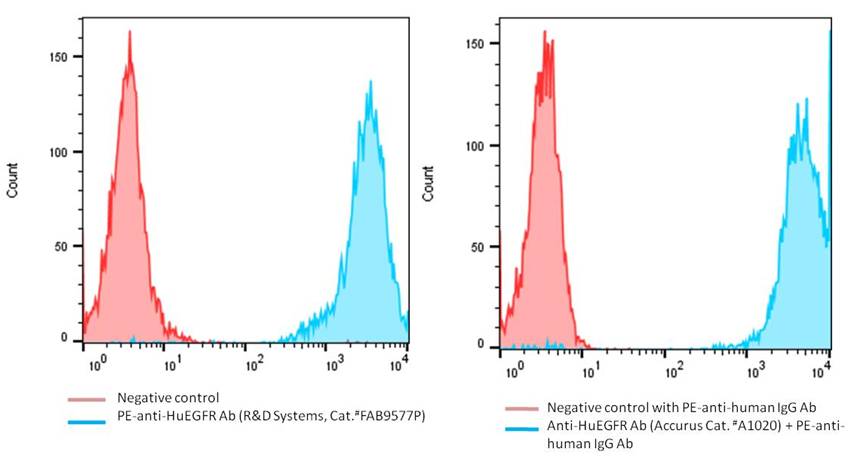
Detection of human EGFR expression on human EGFR-CT26 stable cells using monoclonal antibodies specific for human EGFR.
Epidermal growth factor receptor (EGFR), also known as ErbB-1, is a transmembrane receptor protein that belongs to the receptor tyrosine kinase family. It is encoded by the EGFR gene and is expressed in various tissues, including the epithelial cells of the skin, lung, gastrointestinal tract, and brain. EGFR is involved in several cellular processes, including cell growth, proliferation, differentiation, and survival, through activation of downstream signaling pathways such as the MAPK/ERK and PI3K/Akt pathways. However, dysregulation of EGFR signaling has been linked to cancer development and progression in various cancers, including non-small cell lung, head and neck, colorectal, and pancreatic cancers. Therefore, EGFR has become an attractive therapeutic target in oncology. Small molecular inhibitors, such as gefitinib, erlotinib, and afatinib, and monoclonal antibodies, such as cetuximab and panitumumab, have been developed to target EGFR for the treatment of various cancers, particularly in patients with EGFR mutations or overexpression.
Carpenter G. Annual Review of Biochemistry. 56: 881–914, 1987.
Pai, R., Soreghan, B., Szabo, I. L., Pavelka, M., Baatar, D., Tarnawski, A. S. Nature Med. 8: 289-293, 2002.
Reynolds, F. H., Jr., Todaro, G. J., Fryling, C., Stephenson, J. R. Nature. 292: 259-262, 1981.
Pao, W., Miller, V., Zakowski, M., Doherty, J., Politi, K., Sarkaria, I., Singh, B., Heelan, R., Rusch, V., Fulton, L., Mardis, E., Kupfer, D., Wilson, R., Kris, M., Varmus, H. Proc. Nat. Acad. Sci. 101: 13306-13311, 2004.
Nakamura JL. Expert Opinion on Therapeutic Targets. 11: 463–72. 2007.